is ch3cl polar Ch3cl polarity pole compounds separation charge
When it comes to understanding the properties of molecules, it is essential to determine their polarity. One such molecule is CH3Cl, also known as chloromethane. The question that arises is whether CH3Cl is polar or nonpolar. Let’s delve deeper into this topic and uncover the answer.
Understanding Polarity
Before we get into the specifics of CH3Cl, let’s first review what polarity means. Polarity refers to the distribution of electrical charge within a molecule. If the distribution of electrons in a molecule is uneven, with a higher concentration of electrons on one side, the molecule is considered polar. If the distribution of electrons is even, the molecule is considered nonpolar.
Analyzing CH3Cl
Now that we understand what polarity is let’s analyze CH3Cl. CH3Cl is a tetrahedral molecule with a single carbon atom, three hydrogen atoms, and one chlorine atom. The shape of the molecule means that the bonds are arranged symmetrically around the central carbon atom. However, the electronegativity value of chlorine is higher than that of carbon and hydrogen, causing the electrons to be pulled towards the chlorine atom.
This uneven distribution of electrons means that the molecule is polar, with a partial negative charge on the chlorine atom and a partial positive charge on the hydrogen atoms. This polarity results in the molecule having a dipole moment.
Applications of CH3Cl
Now that we have established that CH3Cl is polar, let’s discuss its applications. CH3Cl is often used as a solvent in the pharmaceutical industry and is used in the production of silicones and quaternary ammonium compounds. It is also used as a refrigerant and is a substitute for chlorofluorocarbons (CFCs) as it is less damaging to the ozone layer.
Safety Concerns of CH3Cl
While CH3Cl is widely used, it is important to note that it can be harmful to humans if precautions are not taken. Prolonged exposure to CH3Cl can cause headaches, confusion, nausea, and even unconsciousness. It is important to use it in a well-ventilated area and to wear protective gear.
Conclusion
In conclusion, CH3Cl is a polar molecule due to the uneven distribution of electrons caused by the electronegativity difference between the atoms. This polarity makes it useful for a variety of purposes, including use as a solvent and refrigerant. However, it is important to use it with caution to avoid harmful effects.
 Figure 1. CH3Cl Polar or Nonpolar
Figure 1. CH3Cl Polar or Nonpolar
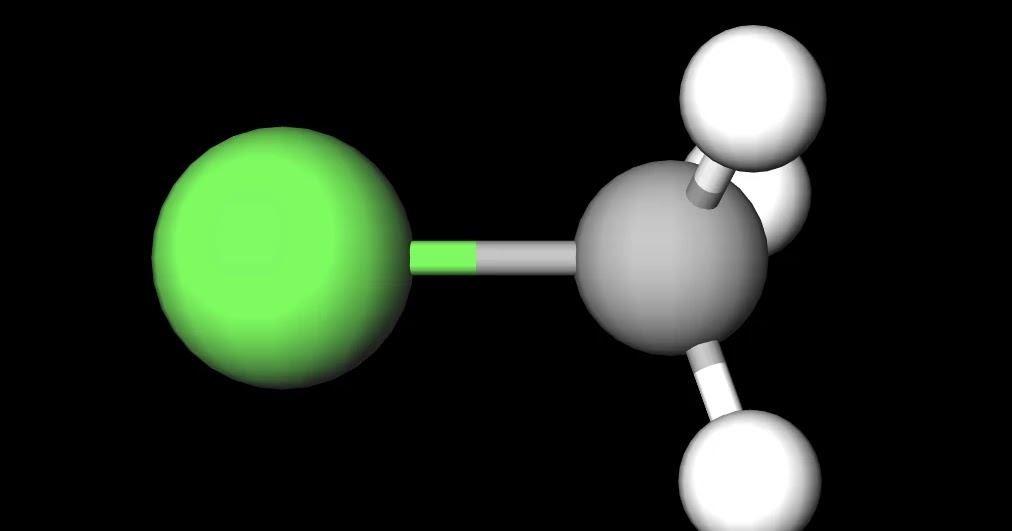 Figure 2. CH3Cl Ball and Stick Model
Figure 2. CH3Cl Ball and Stick Model
If you are looking for Shapes of Molecules you’ve visit to the right page. We have 5 Pictures about Shapes of Molecules like Is CH3Cl Polar or Nonpolar? - YouTube, Is CH3Cl Polar or Non-Polar? - Techiescientist and also Is CH3Cl Polar or Non-Polar? - Techiescientist. Here it is:
Shapes Of Molecules
 www.angelo.edushapes polar molecules lewis polarity molecule vsepr model kboudrea angelo faculty
www.angelo.edushapes polar molecules lewis polarity molecule vsepr model kboudrea angelo faculty
Is CH3Cl Polar Or Nonpolar? - YouTube
 www.youtube.comch3cl polar nonpolar chloride
www.youtube.comch3cl polar nonpolar chloride
Is CH3Cl Polar Or Non-Polar? - Techiescientist
 techiescientist.comch3cl polarity pole compounds separation charge
techiescientist.comch3cl polarity pole compounds separation charge
MakeTheBrainHappy: Is CH3Cl Polar Or Nonpolar?
 www.makethebrainhappy.comch3cl
www.makethebrainhappy.comch3cl
Ch3cl Polar Or Nonpolar - KarissaknoeHanna
 karissaknoehanna.blogspot.comCh3cl polar or nonpolar. Ch3cl. Ch3cl polarity pole compounds separation charge
karissaknoehanna.blogspot.comCh3cl polar or nonpolar. Ch3cl. Ch3cl polarity pole compounds separation charge